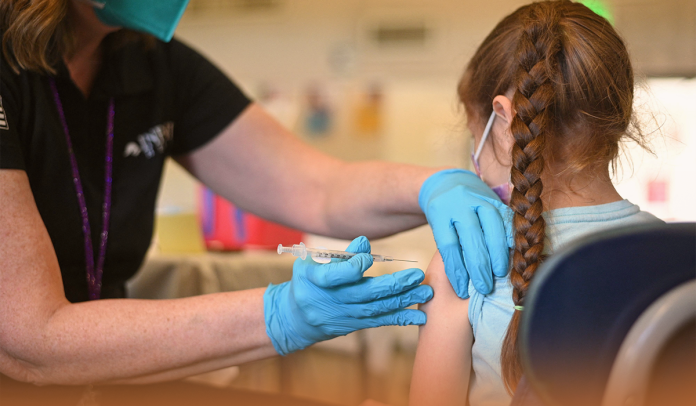
The US Centers for Disease Control and Prevention recently signed off that American kids as young as five-year-olds can now receive the Omicron-tailored Coronavirus booster doses produced by Pfizer and Moderna, a step that would expand the US administration’s Fall inoculation drive.
On October 12, the U.S. FDA granted approval for using Pfizer’s updated vaccine dose in Americans aged five and up, while Moderna’s bivalent shot got the ‘OK’ in US kids aged six-year and up. Moreover, the CDC supported the FDA’s approval, smoothing the path for the doses to be used in kids.
In addition, the restructured bivalent shots from Pfizer and Moderna target the original strain as well as the highly contagious Omicron-subvariants, labeled as BA.4 & BA.5. According to the US David Heath, a Sacramento, California-based nationally renowned academic medical center, currently, BA.5 subvariant of Omicron has become the most dominant strains of the lethal Coronavirus in America, accounting for about 79 percent of the US cases. It’s able to escape protection from Coronavirus infection and immunization.
Peter Marks, Director – CBER at the FDA, said that since American children have resumed their schooling and people are returning to pre-COVID activities, there’s the possibility for increased risk of contracting the highly infectious virus. Immunization remains the most productive way to get protected from the severe consequences of the Coronavirus disease, including hospital admission and unfortunate fatality.
How many Americans got the updated vaccine doses?
According to the US health regulators, overall, the Coronavirus immunization rates have remained low among children, with around forty percent of Americans ages 5-11-years protected with a single shot of the jab so far. So, Marks encouraged the parents of children five-year-old and above to consider immunization for them and continue with a modified shot when they become eligible.
Source: Web
Pfizer, a US multinational pharmaceutical company headquartered in Manhattan, New York City, said it could deliver around 6M pediatric shots in the first week after CDC’s recommendation without affecting the distribution output of the vaccine shots for 12-year-olds and up.
And according to US health officials, nearly 11.5M Americans received the updated doses since the rollout in September, representing only 5.4 percent of eligible Americans among 12-year-olds and up.
Read Also: US CDC Will No Longer Maintain Country-Specific Travel Notices